Description
Description
1. BOSS is a xenograft using bovine cancellous bone and its main ingredient is HA (Hydroxyapatite).
2. Pores in this material are interconnected, which facilitates the formation of new bone
tissue into the material when implanted in oral and maxillofacial bone.
3. During the manufacturing process, the organic components were completely removed
through chemical treatments and heat treatment.
4. BOSS should be used by trained/qualified licensed persons familiar with bone grafting
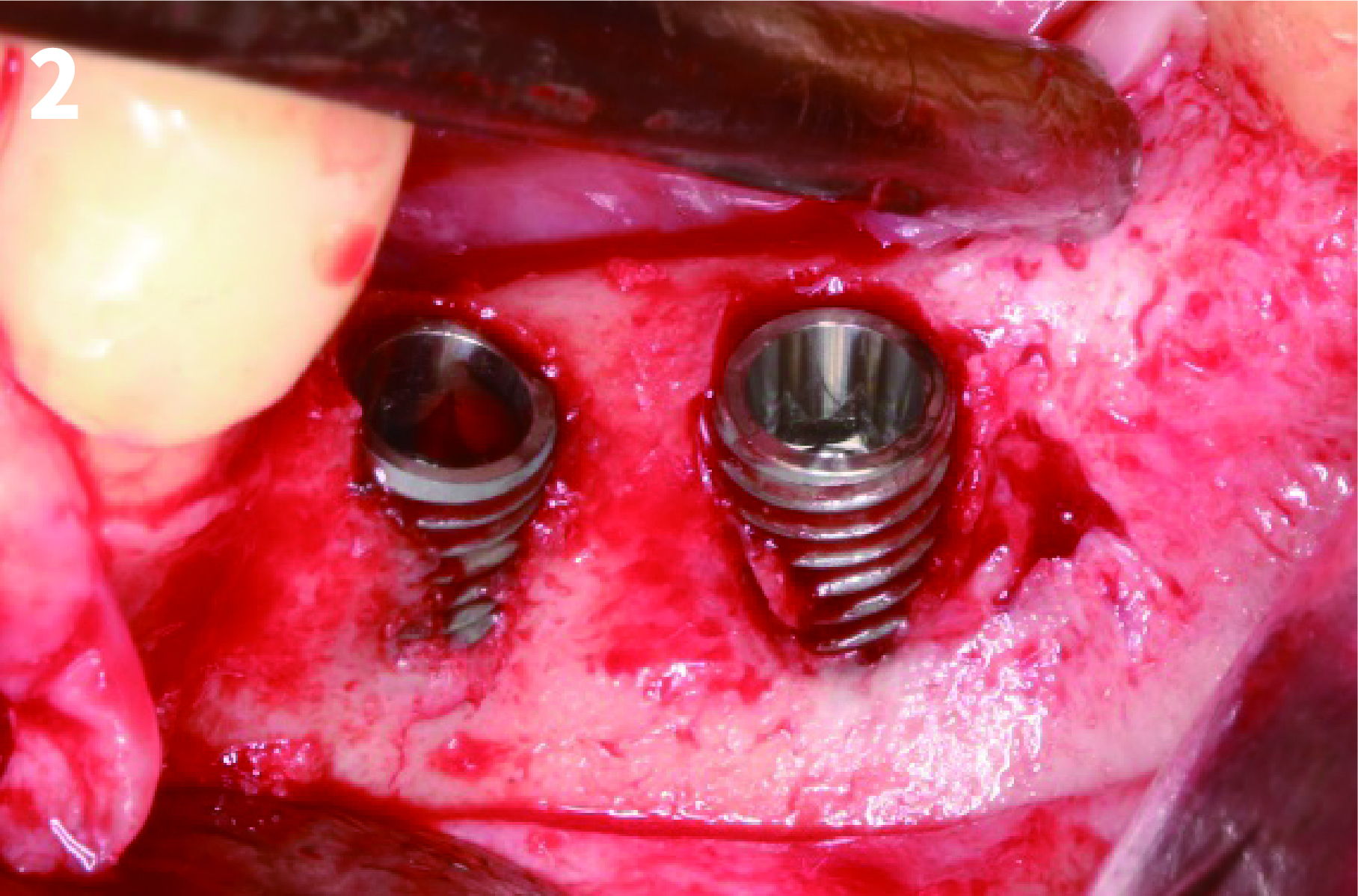
Case 1: Simply Augmented Severe Dehiscence Defect on Anterior Area
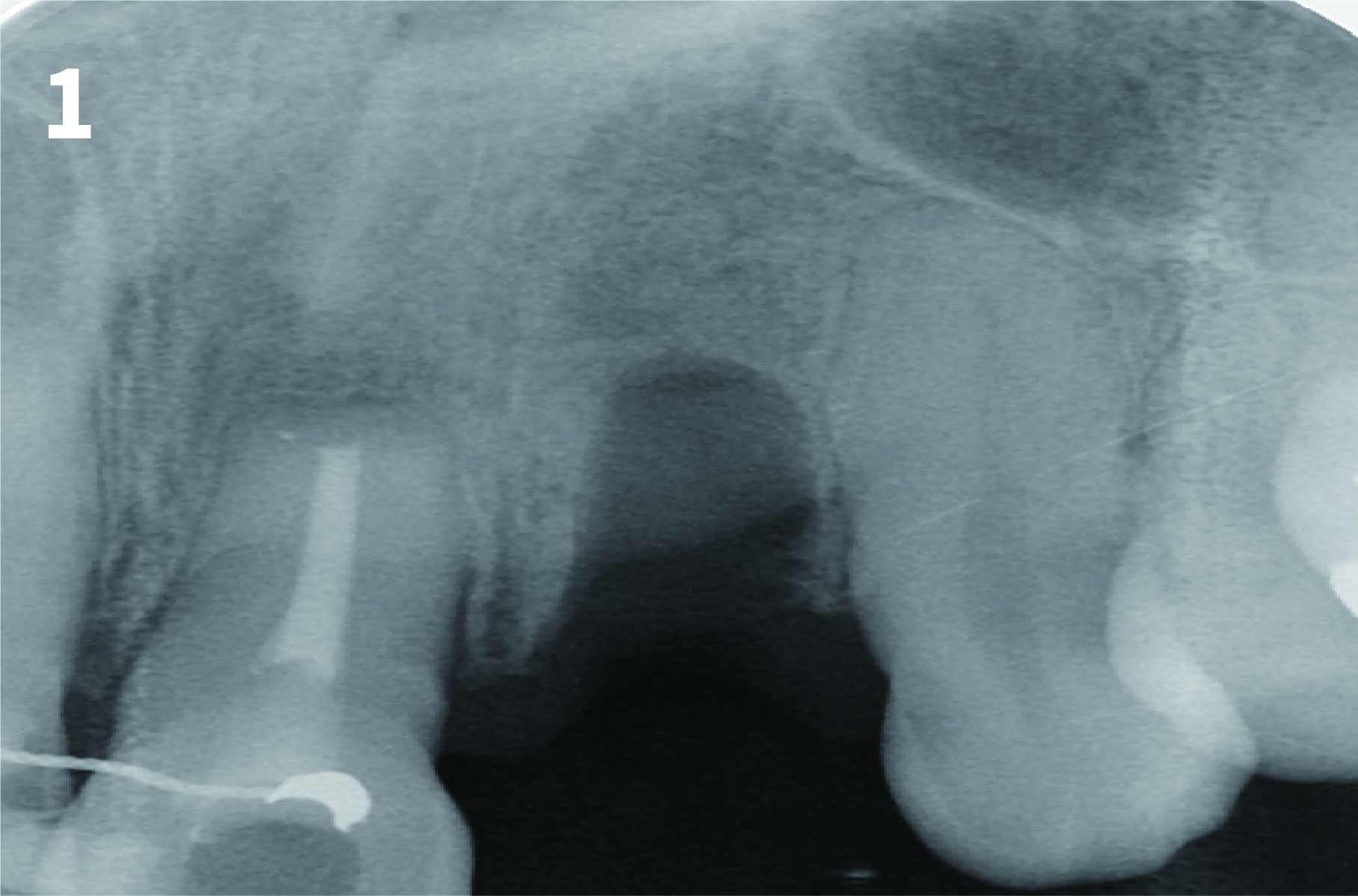
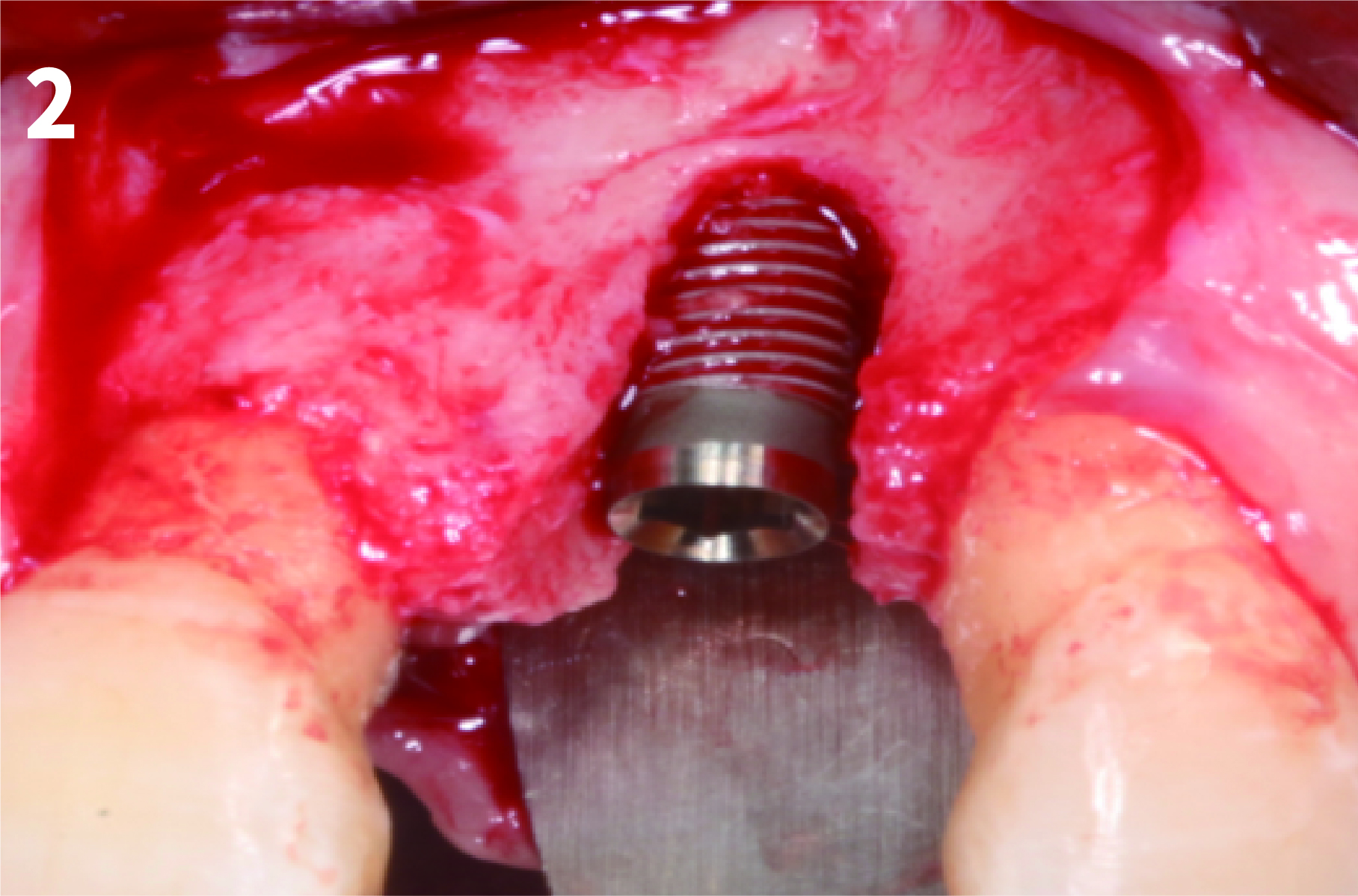
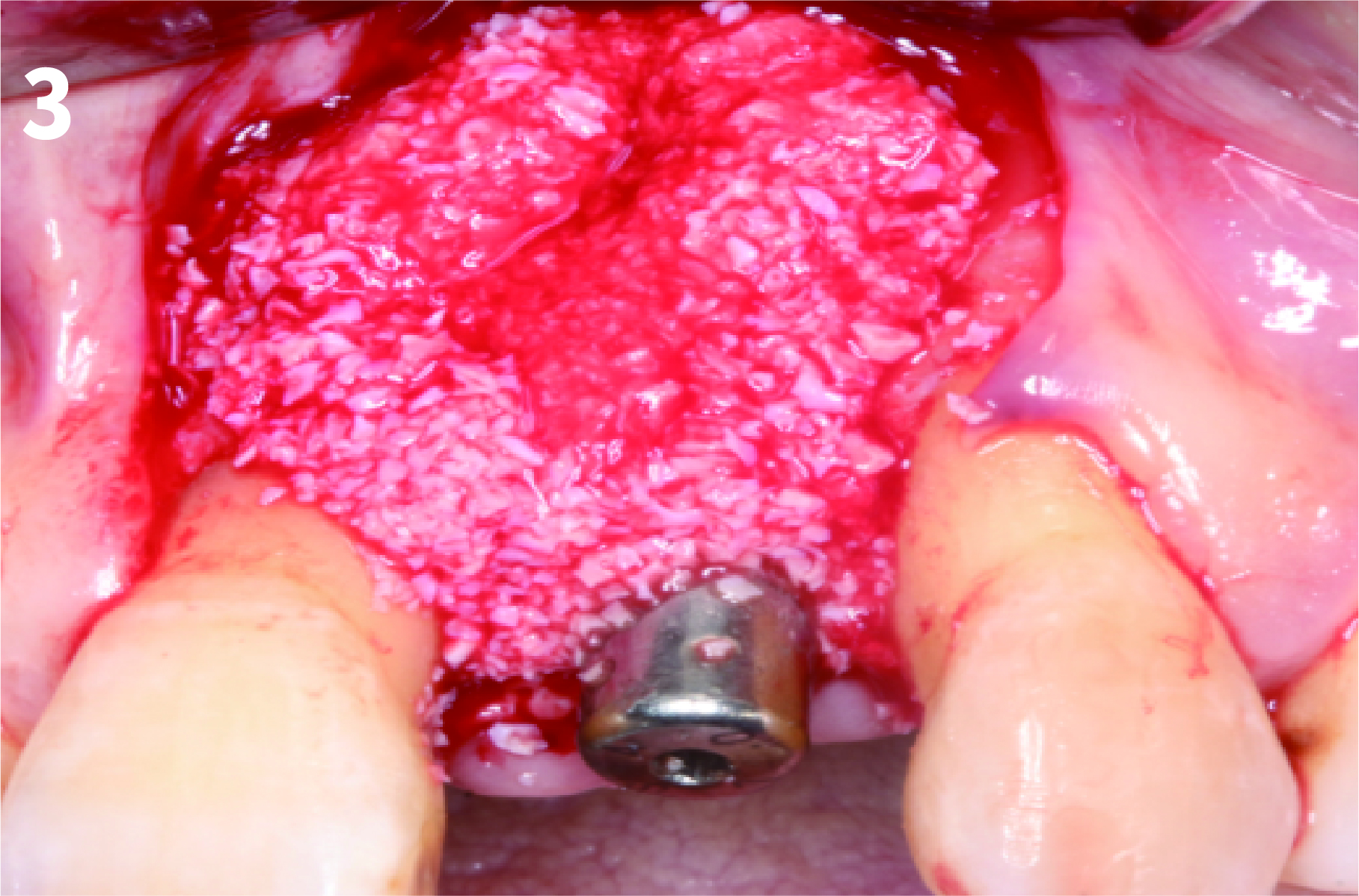
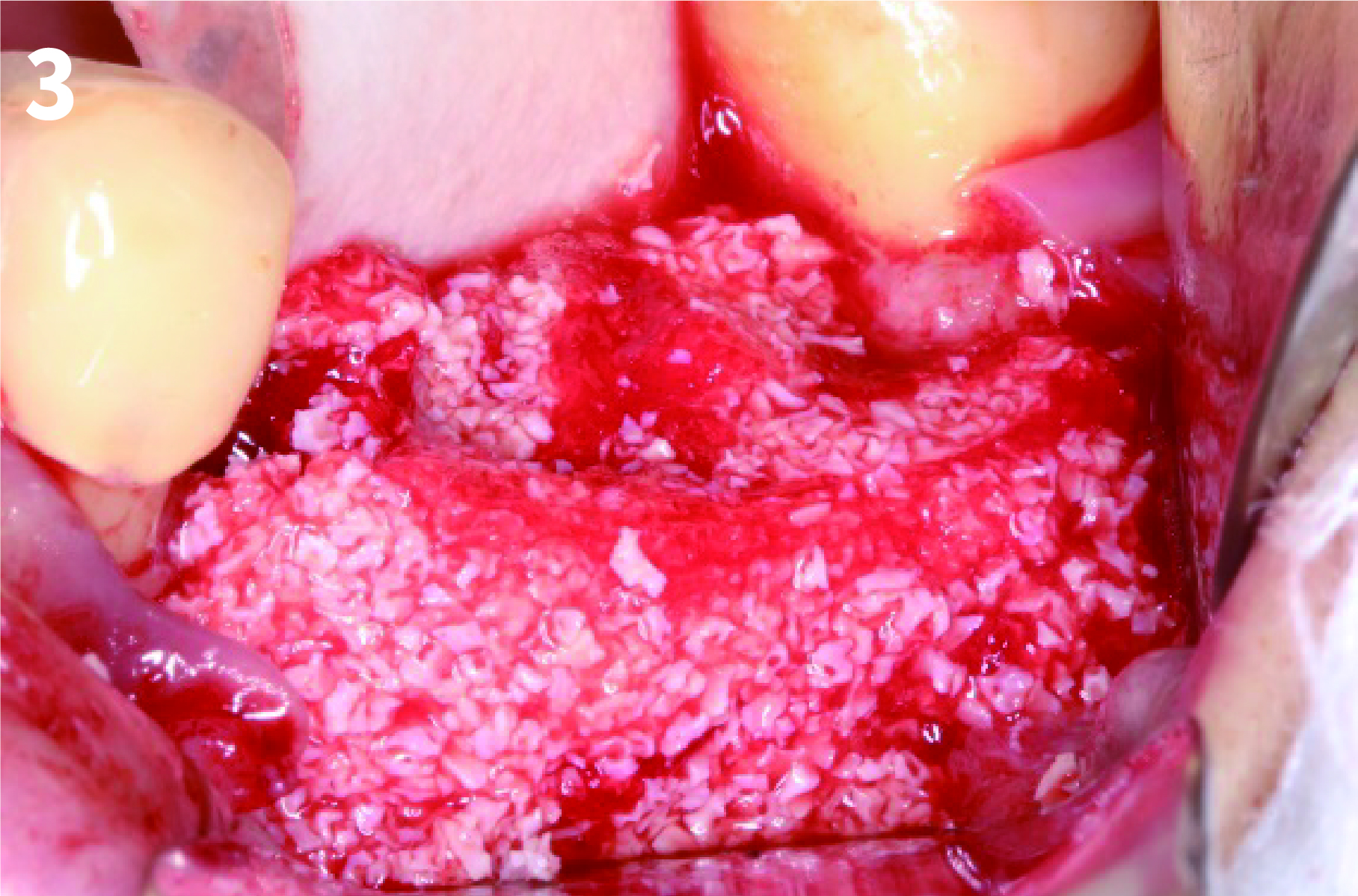
Case 2: Minor Bone Augmentation
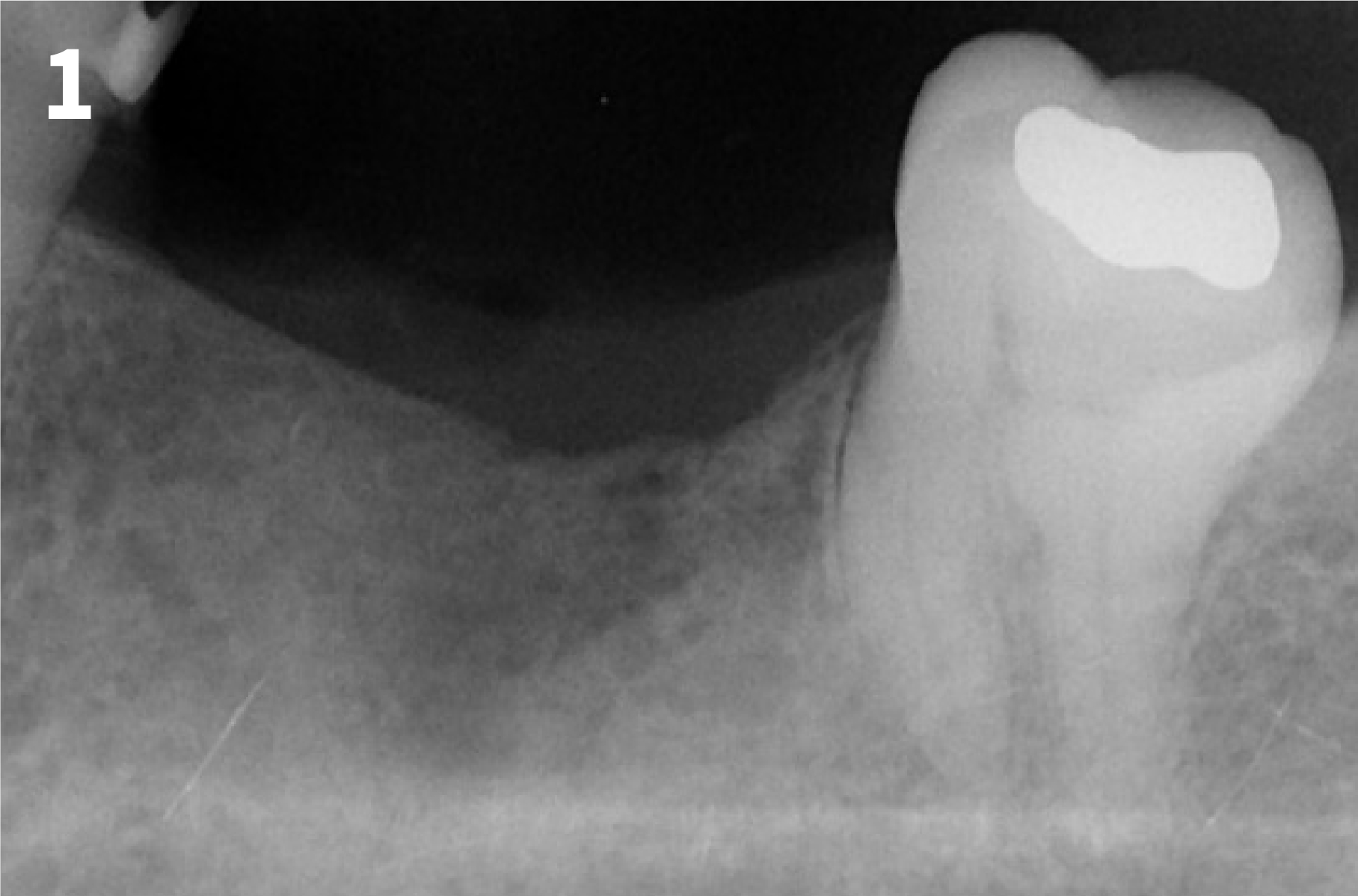
Intended Use
MedPark BOSS is a xenograft material using bovine cancellous bone for the purpose of securing a space where a new bone tissue is formed by filling defects or bony voids of the oral and maxillofacial region due to a surgical injury or a non-surgical injury.
Preoperative Preparation
1. Deliver a double packed product to the operating room while keeping it sterilized.
2. Do not use BOSS if the sterile package is damaged or opened.
3. Do not use it if any foreign materials are found inside the vial.
4. Do not use BOSS bone graft after the expiration date.
The validity period is 3(three) years from the date of manufacture.
5. Read and fully understand 'Directions for Use' before surgery and be sure that they know exactly how to use BOSS.
6. The surgical instruments must be sterilized before the operation.
7. BOSS is an implantable medical device and it should be used in a clean environment, operating room.
Directions for Use
The general principles of sterile handling and patient medication must be followed when using BOSS.
1. When using it, peel off blister Tyvek film. Holding BOSS vial firmly, remove the cap. Dispense the granules of BOSS into a sterile container.
2. After exposure the bony defect with mucoperiosteal flap, completely remove the granulation tissue, inflammatory tissue.
3. When opening the sterile package, never store remained product after.
4. After wetting the BOSS granules, fill up the defect area with the bone graft.
5. Xenograft materials can be mixed with autogenous bone at the discretion of a clinician.
6. After complete wetting the granulate of BOSS in saline solution, remove any excess fluid, it is filled into the bone defect. The graft material should be treated carefully neither to damage nor to change the transplant material.
7. After filling the graft, cover the surgical site with mucoperiosteal flap and should be fixed by sutures. Be sure to completely seal the implantation site to prevent exposure.
- Fundamentally, the use of powder type is recommended for small defects (up to 2 dental alveoli) and for augmenting autogenous grafts. The chip type are recommended for large defects (> 2 dental alveoli, sinus lifts), however, preferences can vary from dentist to dentist.Powder and Chip type can be mixed together at the dentist's discretion.
Storage and Expiration date
This product is supplied in a sterile container. Store as it is at room temperature(1℃ to 30℃) in the shade before use. If the sterile packaging is damaged or opened, the product must not be used. The contents of the blister package or vial are designed for single use only. Discard any unused material after opening. Expiration date is 3 years from date of manufacture. Do not use and re-sterilize products that have expired.
Precautions
1. Warnings
1) Discard any unused material after opening. Never reuse! 2) Do not use if package is opened or damaged or if expiration date has been exceeded. 3) This product should only be used by trained dentists or a oral surgeons.
2. Patients with the following diseases are prohibited.
1) Patients with osteomyelitis 2) Patients with severe liver dysfunction 3) Patients with severe cardiac dysfunction.
3. Side Effect
Incompatibility reactions with MedPark BOSS cannot be totally excluded. Possible complications which may occur with any surgery include swelling at the surgical site, flap sloughing, bleeding, local inflammation, bone loss, infection, or pain.
4. General precautions
1) In general, the conditions considered as standard contraindications for bone graft are metabolic diseases, osteoporosis, steroid therapy, autoimmune diseases, nicotinism. 2) For bone regeneration, the product is only implantable to bone tissue that is directly connected to living bone tissue and host bone. In the case of large injuries, the addition of autogenous cancellous bone or bone marrow can improve the regeneration process.Experience has shown that movement due to increased physical loads (compression loads) or implantation of implants (2-step procedures) should be avoided until several weeks after insertion of the product. Experiments have shown that the physical loading (compression load) of this product augmented areas is possible after 6 months at the earliest. Implant placement time is determined by the amount of remaining local bone.
5. Pregnant and lactating women, infants, children
1) Do not use BOSS for pregnant or lactating women. 2) It should not be used in patients who are skeletally immature. (<18 years of age of no radiographic evidence of epiphyseal closure)
6. Application notes
1) Do not leave any parenchyma or other soft tissue on the defective area to be implanted. 2) This product has a low mechanical strength, so be careful when filling the contents. 3) Do not overfill defective or surgical sites. 4) The defect site and the surgical site must be completely closed.
7. Others
1) One patient can be implanted with a maximum of 2.5 g BOSS during one procedure and their lifespan. 2) It is recommended to use the amount corresponding to the defect within the maximum allowable dosage. The amount of the product can be determined at the discretion of the dentist.
Technical Data
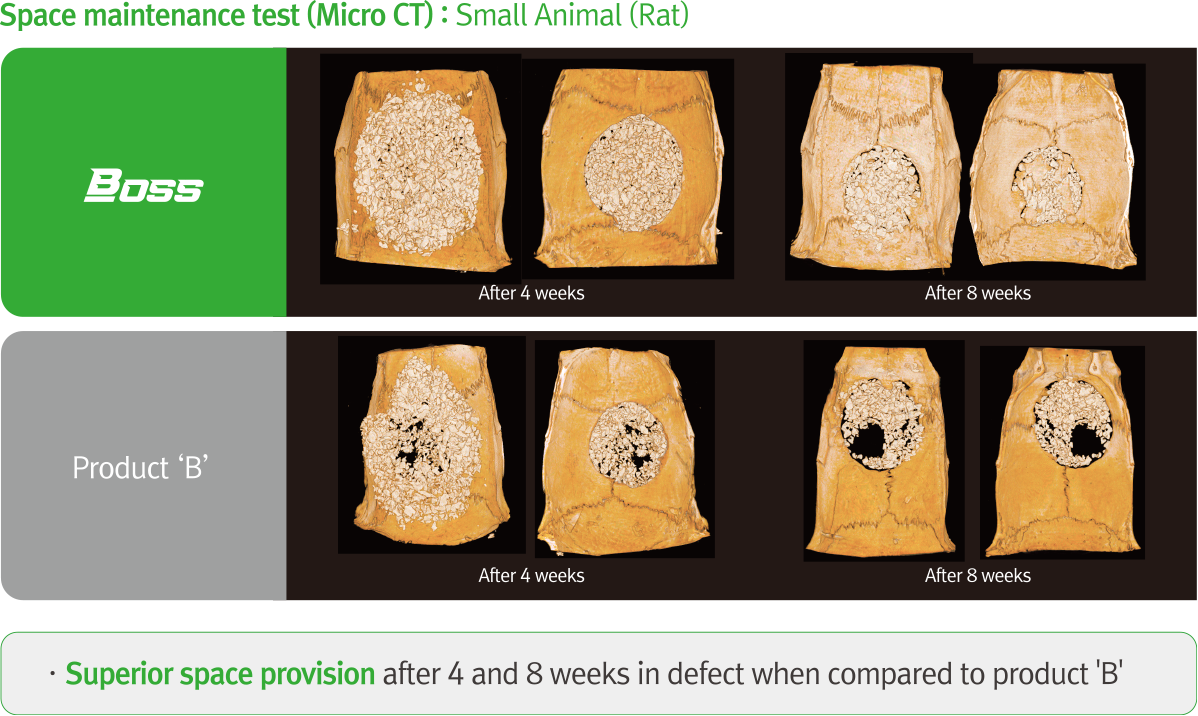







-thumb_270.jpg)








0 Review for “Bovine Bonegrafts | Natural Bone Substitute”
5 Stars
0%
4 Stars
0%
3 Stars
0%
2 Stars
0%
1 Stars
0%